Bacillus clausii
Bacillus clausii PBC429 (MTCC25459)
Bacillus clausii renamed as Shouchella clausii1 is gram positive non-pathogenic spore forming probiotic, it is safe for human consumption. It produces antimicrobial proteins and is stable at a wide range of processing conditions without losing its viability. Bacillus clausii PBC429 submitted to genebank with accession number MTCC 25459. Bacillus clausii is in QPS list for EU2.
Bacillus clausii PBC429 benefits
- Bacillus clausii is useful in diarrhea.
- Inhibit the growth of pathogens in the intestinal tract.
- Promotes a healthy gut microbiome.
- Can withstand harsh environments like acidic pH, high temperature.
- Stable at room temperature does not require refrigeration.
- Maintains normal bowel function.
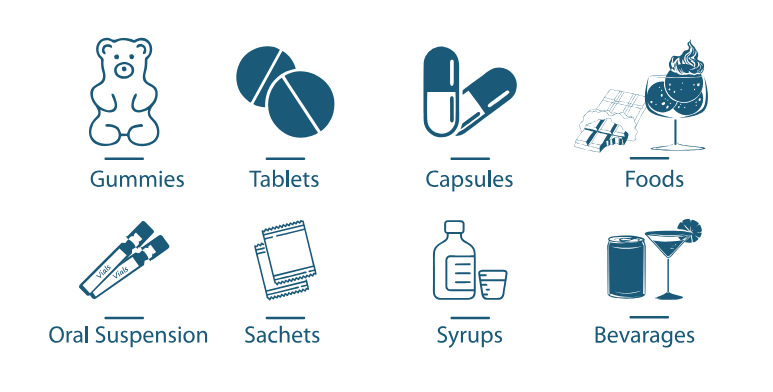
Application
Nutraceuticals
B. clausii PBC429 can also be used in various dosage form such as oral suspension, tablet, capsule, gummies, sachets in nutraceutical application.
Foods
B. clausii PBC429 can be used in a range of functional foods and beverages, such as ice and hot tea, juices, instant coffee, flavored drink, sports drink, yogurt, cereal bars breakfast cereals, and ice creams, chocolates, ready mix soup, instant noodles, bakery products, ketchup, etc.
Features
- Spray dried Product Bacillus clausii is manufactured in FSSC 22000 certified GMP manufacturing facility.
- Manufactured using no animal origin components and is Kosher and Halal certified.
- Stable through a wide range of pH and temperature.
- Resistant to bile salt.
- Stable in various matrix.
- Allergen-Free
Availability & Packaging
- Bulk powder available in potency of 200, 150 Billion CFU/g.
- Blended product available in potency of 100, 50, 25, 15, 6 Billion CFU/g.
- Packaging in Double Polyethylene liners in HDPE Drums or foil bags.
- Foil Bags: 25 kgs, 20 kgs, 10 kgs, 5kgs, 1Kg in foil bags
- Drum: 25 kgs and 20 kgs.
Documents
- Microbial Identification Report – 16S rRNA
- Whole Genome Sequence Report
- Certificate of Analysis (COA)
- Material Safety Data Sheet (MSDS)
- Stability Data
References
- Alkalihalobacterium elongatum gen. nov. sp. nov.: An Antibiotic-Producing Bacterium Isolated From Lonar Lake and Reclassification of the Genus Alkalihalobacillus Into Seven Novel Genera. Front Microbiol. 2021 Oct 11;12:722369. doi: 10.3389/fmicb.2021.722369.
- Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. The EFSA Journal (2007) 587, 1-16
Certificate
Licences and Certificates







