Bacillus subtilis
Bacillus subtilis BSP110 (MTCC 25471)
Bacillus subtilis is a member of the Bacillus genus and is a rod-shaped, gram-positive bacteria found in the soil and gastrointestinal tract of ruminants and humans. it is safe for human and animal consumption. it forms a tough endospore that helps to withstand extreme environmental conditions, it form biofilms which enhance gut colonization. Bacillus subtilis BSP110 submitted to gene bank with accession number MTCC 25471. It is in QPS list for EU1.
Bacillus subtilis BSP110 Benefits
- Maintains micro-flora of the gut thus improving the healthy gastrointestinal health.
- Helps in antibiotic associated diarrhoea.
- Highly stable and resilient spores sustain gastric condition.
- Produce bacteriocins.
- Produce enzymes such as amylase and lipase important for carbohydrate and lipid metabolism.
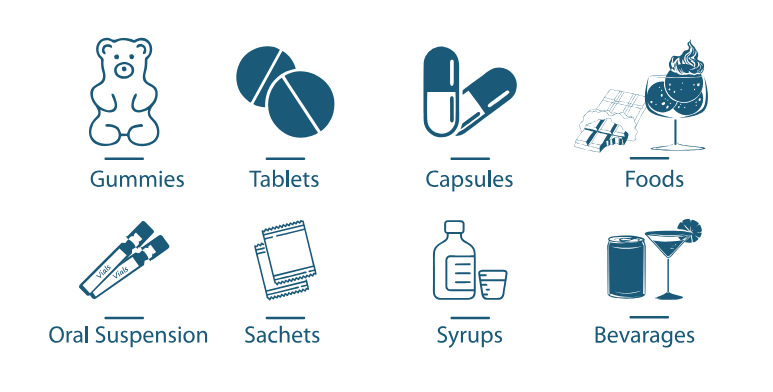
Application
Nutraceuticals
B. subtilis BSP110 can be in various dosage form such as oral suspension, tablet, capsule, gummies, sachets in nutraceutical application.
Foods
B. subtilis BSP110 can be used in a range of functional foods and beverages, such as ice and hot tea, juices, instant coffee, flavored drink, sports drink, yogurt, cereal bars breakfast cereals, and ice creams, chocolates, ready mix soup, instant noodles, bakery products, ketchup, etc
Features
- Spray dried Product Bacillus subtilis is manufactured in FSSC 22000 certified GMP manufacturing facility.
- Manufactured using no animal origin components and is Kosher and Halal certified.
- Stable through a wide range of pH and temperature.
- Resistant to bile salt.
- Stable in beverages such as tea, coffee, and energy drinks.
- Stable in baked goods and energy bars.
- Allergen-Free.
Availability & Packaging
- Bulk powder available in potency of 200, 150 Billion CFU/g.
- Blended product available in potency of 100, 50, 25, 15, 6 Billion CFU/g.
- Packaging in Double Polyethylene liners in HDPE Drums or foil bags.
- Foil Bags: 25 kgs, 20 kgs, 10 kgs, 5kgs, 1Kg in foil bags
- Drum: 25 kgs and 20 kgs.
Documents
- Microbial Identification Report – 16S rRNA
- Whole Genome Sequence Report
- Certificate of Analysis (COA)
- Material Safety Data Sheet (MSDS)
- Stability Data
References
- Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. The EFSA Journal (2007) 587, 1-16
Certificate
Licences and Certificates







